Pharmaceutical and Sharps Waste Stewardship
California’s Pharmaceutical and Sharps Waste Stewardship Programs provide safe and convenient disposal options for pharmaceutical and home-generated sharps waste at no cost to the consumer. CalRecycle oversees the programs, which are run by MED-Project and The Drug Takeback Solutions Foundation, the nonprofit stewardship organizations created by pharmaceutical and sharps manufacturers and other entities within the distribution chain.
Program News
- On January 26, 2026, The Drug Takeback Solutions Foundation notified CalRecycle it is terminating its stewardship plans for covered drugs and home-generated sharps waste on June 30, 2026.
- On December 11, 2025, MED-Project notified CalRecycle of proposed changes to its stewardship plan for home-generated sharps waste regarding its mail-back program.
- On October 23, 2025, The Drug Takeback Solutions Foundation notified CalRecycle of proposed changes to its stewardship plan for covered drugs regarding reasonable geographic spread and supplemental mail-back service.
- On January 27, 2026, The Drug Takeback Solutions Foundation rescinded these proposed changes.
Read the law and regulations for details.
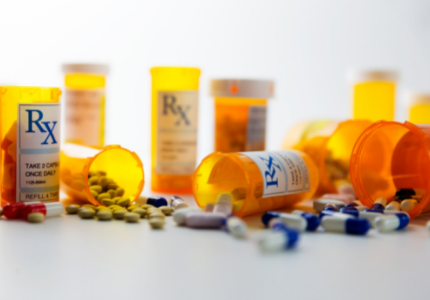
Where and How Do I Dispose of Pharmaceuticals?
To find disposal drop-off locations or obtain mail-back supplies, visit medtakebackcalifornia.org or call (844) 4-TAKE-BACK or (844) 482-5322.
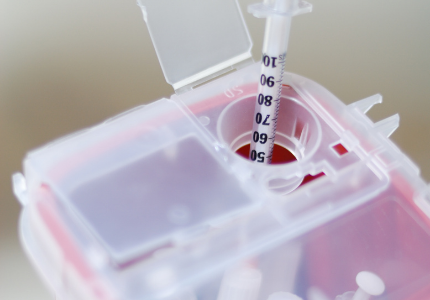
How Do I Dispose of Home-Generated Sharps Waste?
To order a free sharps waste container with mail-back materials, visit sharpstakebackcalifornia.org or call (844) 4-TAKE-BACK or (844) 482-5322.

More Information
- CalRecycle Public Notices page
- To report a suspected issue, use the pharma sharps referral system.
- Contact PharmaSharps@calrecycle.ca.gov for questions or more information.
This page is not intended to provide legal determinations or contain a comprehensive list of legal requirements.
- Contact the Board of Pharmacy at BOPstewardship@dca.ca.gov for questions or to submit information pursuant to PRC 42031 and 42032(b)(1).
- Contact the California Department of Public Health at MedicalWaste@cdph.ca.gov for medical waste questions.
- Visit the California Department of Toxic Substances Control Pharmaceuticals and Sharps webpage and contact PharmStewardship@dtsc.ca.gov for questions about hazardous waste management.
